问题:
(1)已知:H 2(g)+O 2(g)  H 2O(g),反应过程中能量变化如图所示,则:
H 2O(g),反应过程中能量变化如图所示,则:
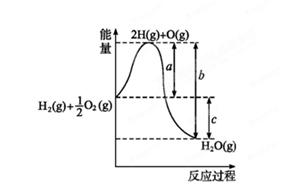
①试写出a、b、c分别代表的意义:
a ;
b ;
c 。
②该反应是 反应(填“吸热”或“放热”),ΔH 0(填“>”或“<”)。
(2)发射“天宫”一号的火箭使用的推进剂是液氢和液氧,这种推进剂的优点是 , 。(请写两条)
(3)已知:H 2(g)+ 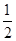 O 2(g)=H 2O(l) ΔH="-285.8" kJ·mol -1
O 2(g)=H 2O(l) ΔH="-285.8" kJ·mol -1
H 2(g)==H 2(l) ΔH="-0.92" kJ·mol -1
O 2(g)==O 2(l) ΔH="-6.84" kJ·mol -1
H 2O(l)=H 2O(g) ΔH="+44.0" kJ·mol -1
请写出液氢和液氧生成气态水的热化学方程式: 。
(1)已知:H 2(g)+O 2(g)  H 2O(g),反应过程中能量变化如图所示,则:
H 2O(g),反应过程中能量变化如图所示,则:

①试写出a、b、c分别代表的意义:
a ;
b ;
c 。
②该反应是 反应(填“吸热”或“放热”),ΔH 0(填“>”或“<”)。
(2)发射“天宫”一号的火箭使用的推进剂是液氢和液氧,这种推进剂的优点是 , 。(请写两条)
(3)已知:H 2(g)+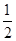 O 2(g)=H 2O(l) ΔH="-285.8" kJ·mol -1
O 2(g)=H 2O(l) ΔH="-285.8" kJ·mol -1
H 2(g)==H 2(l) ΔH="-0.92" kJ·mol -1
O 2(g)==O 2(l) ΔH="-6.84" kJ·mol -1
H 2O(l)=H 2O(g) ΔH="+44.0" kJ·mol -1
请写出液氢和液氧生成气态水的热化学方程式: 。
 H 2O(g),反应过程中能量变化如图所示,则:
H 2O(g),反应过程中能量变化如图所示,则: 
①试写出a、b、c分别代表的意义:
a ;
b ;
c 。
②该反应是 反应(填“吸热”或“放热”),ΔH 0(填“>”或“<”)。
(2)发射“天宫”一号的火箭使用的推进剂是液氢和液氧,这种推进剂的优点是 , 。(请写两条)
(3)已知:H 2(g)+
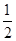 O 2(g)=H 2O(l) ΔH="-285.8" kJ·mol -1
O 2(g)=H 2O(l) ΔH="-285.8" kJ·mol -1 H 2(g)==H 2(l) ΔH="-0.92" kJ·mol -1
O 2(g)==O 2(l) ΔH="-6.84" kJ·mol -1
H 2O(l)=H 2O(g) ΔH="+44.0" kJ·mol -1
请写出液氢和液氧生成气态水的热化学方程式: 。
参考答案:
